Draw All Resonance Structures for the Nitryl Fluoride Molecule No2f
Chemistry questions and answers. Who are the experts.
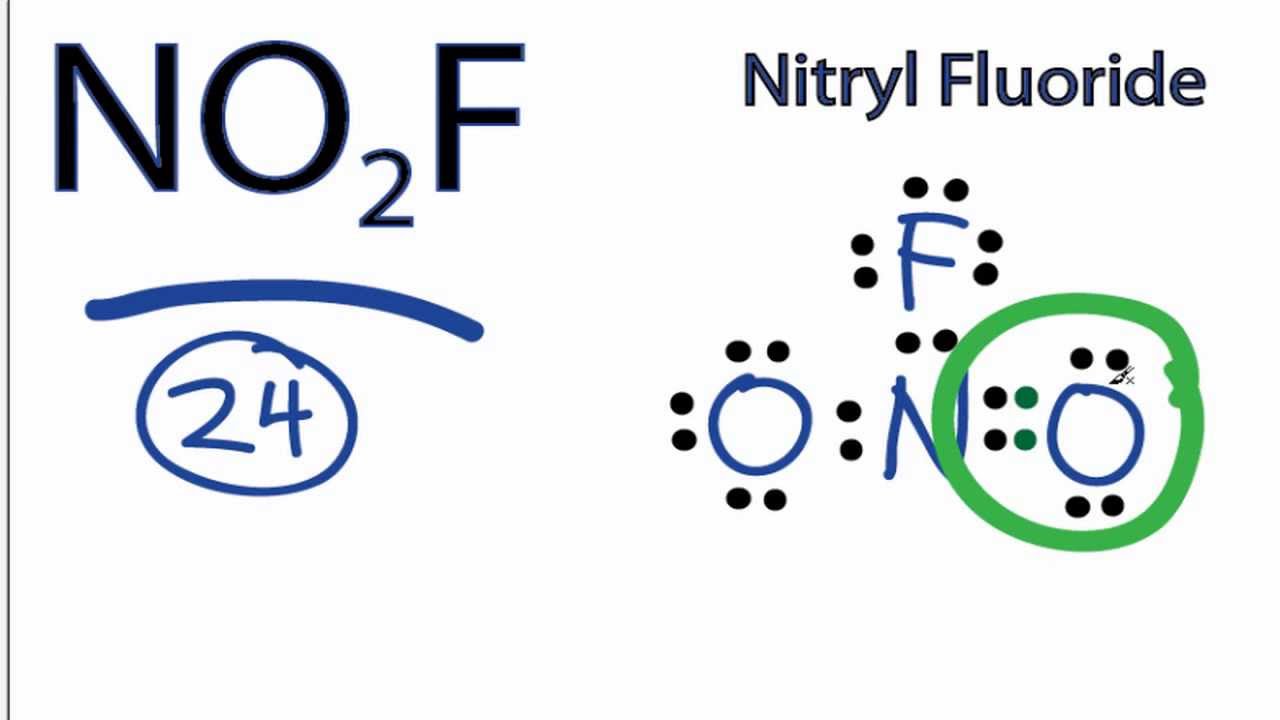
No2f Lewis Structure How To Draw The Lewis Structure For No2f Youtube
Nitryl Fluoride NO2F Molecular Geometry Polarity.
. Here we are going to learn about the chemical bonding of Nitryl Fluoride. NO2F - Nitryl Fluoride. Here NO2 is oxidized to NO2F and CoF3 is reduced to CoF2.
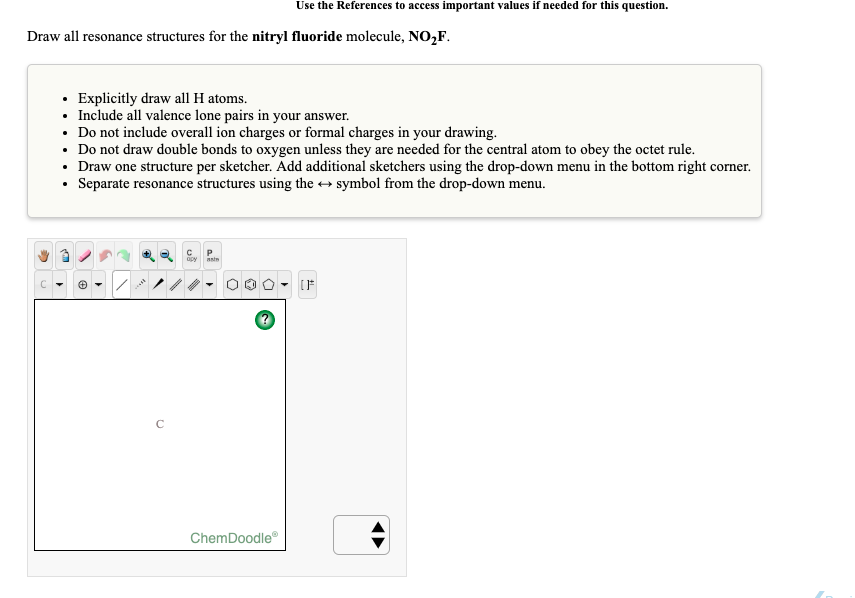
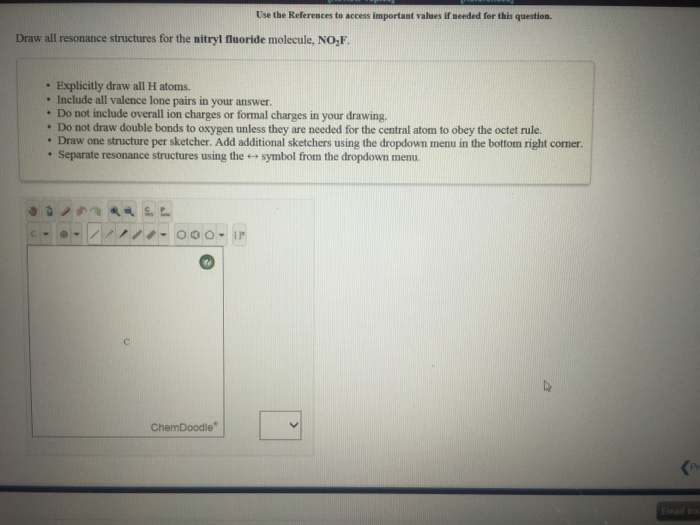
See the answer See the answer done loading. Draw all resonance structures for the nitryl fluoride molecule NO2F. Perhaps that principle is worth reinforcing with students.
Therefore NO2F is polar. Draw one structure per sketcher box and separate any added sketcher boxes with the symbol. The molecular weight of nitryl fluoride is 65 gmol.
Of course this would also be an interesting computational chemistry exercise comparing the relative. For molecules and ions we can draw several resonance structures and their stability is different from one structure to another structure and you should have the ability to identify stability of each structure. Calculate the of electrons in π bonds pi bonds multiple bonds using formula 1.
Draw all resonance structures for the nitryl fluoride molecule NO2F. Draw all resonance structures for the nitryl fluoride molecule NO2F. The structure features planar nitrogen with a short N-F bond length of 135 pm.
Connect the atoms with single bonds. The chain form of NO2F possesses no formal charges but the central N structure should have resonance stabilization. Nitryl fluoride NO 2 F is a colourless gas and strong oxidizing agent which is used as a fluorinating agent and has been proposed as an oxidiser in rocket propellants though never flown.
The structure features planar nitrogen with a short N-F bond length of 135 pm. Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. Connect the atoms of NO2Cl with single bonds.
When we drew lewis structure of NO 2-ion we can draw resonance structures of NO 2-ion. In this tutorial you can see how many resonance structures can be drawn for nitrite ion NO 2- and what are the steps you need to knowAlso we will discuss which resonance structures are more stable an which are less stable. NO 2-Resonance Structures Nitrite ion.
By signing up youll get thousands of step-by-step solutions to. Do not include overall ion charges or formal charges in your drawing Do not draw double bonds to oxygen unless they are needed for the central. There are rules to follow drawing resonance structures step by step.
Experts are tested by Chegg as specialists in their subject area. Let us consider the case of nitryl chloride NO2Cl. Draw all resonance structures for the nitryl fluoride molecule NO2F.
A step-by-step explanation of how to draw the NO2F Lewis Structure. The molecular geometry of NO 2 F is trigonal planar with asymmetric charge distribution on the central atom. Up to 256 cash back draw all resonance structures for the nitryl chloride molecule no2cl.
Draw all resonance structures for the nitryl fluoride molecule no2f. Explicitly draw all H atoms. Well put a pair of electrons between atoms to form chemical bonds and then well go around the outside until we use 24 valence electrons.
Where n in this case is 4. Draw all resonance structures for the nitryl fluoride molecule NO2F. Do not include overall ion charges or formal charges in your drawing.
Include all valence lone pairs in your answer. Up to 256 cash back OneClass. First draw the Lewis dot structure.
NO2 CoF3 NO2F CoF2. It is a molecular species not ionic consistent with its low boiling point. The central atom is the N atom.
A step-by-step explanation of how to draw the NOF2 Lewis Dot Structure Nitryl fluoride For the NOF2 structure use the periodic table to find the total num. Draw all resonance structures for the nitryl fluoride molecule NO2F. Nitrogen is the least electronegative well put that at the center put the Oxygens on the outside and the Fluorine as well.
Explicitly draw all H atoms. Now every atom has eight electrons but nitrogen can form only three bonds owing to the absence of d-orbitals. Structures having multiple resonable resonance forms may be more stable.
Draw one structure per sketcher. For the NO2F Lewis structure we have a total of 24 valence electrons. There are equivalent Lewis structures for NO_2F.
Draw all resonance structures for the nitryl fluoride molecule NO2F. The nitryl fluoride is prepared by fluorination of nitrogen dioxide NO2 by cobalt trifluoride CoF3. NO 2 F.
Nitryl fluoride NO2F is a colourless gas and strong oxidizing agent which is used as a fluorinating agent and has been proposed as an oxidiser in rocket propellants. Do NOT show any ion charges in your drawings. Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule.
Draw all resonance structures for the nitryl fluoride molecule NO2Fa Explicitly draw all H atomsb Include all valence lone pairs in your answerc Do not include overall ion charges or formal charges in your drawingd Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rulee Draw one structure per sketcher. We review their content and use. How to Draw Resonance Structures Rules Examples Problems.
Chemistry questions and answers Draw all resonance structures for the nitryl fluoride molecule NO2F. Nitryl fluoride FNO2 CID 66203 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Include all valence lone pairs in your answer.
Then draw the 3D molecular structure using VSEPR rules. For Nitryl Chloride NO2Cl the total number of valence electrons available is 245 from nitrogen 7 from chlorine 62 from 2 oxygen atoms. Draw Lewis structures showing all possible equivalent resonance forms for the nitryl fluoride molecule NO 2 F.
Solved Draw All Resonance Structures For The Nitryl Fluoride Chegg Com

Draw All Resonance Structures For The Nitryl Fluoride Molecule No2f A Explicitly Draw All H Brainly Com

Solved Use The References To Access Important Values If Chegg Com
Comments
Post a Comment